Platinum: Beyond Precious - Unveiling the Mystique of the Noble Metal
Platinum:
Platinum, a noble metal with a rich history and undeniable allure, has captivated humanity for centuries. Its remarkable properties and versatility have made it a sought-after element in various industries and adornments. In this blog, we will delve into the fascinating world of platinum, exploring its chemical characteristics, applications, and the glamour it adds to jewelry.
Chemical Properties:
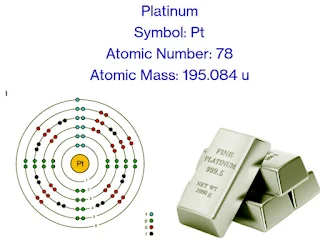
Symbol: Pt
Atomic Number: 78
Atomic Mass: 195.084 u
Position in the Periodic Table: Group 10, Period 6
Electron Configuration: [[Xe] 4f14 5d9 6s1
or Electron Configuration in long form: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d9 6s1
Valency: +2, +4, +6
Chemical and Physical Properties:
Platinum boasts an impressive array of properties, including high melting and boiling points, excellent corrosion resistance, and remarkable catalytic activity. Its malleability and ductility make it easy to work with, while its lustrous appearance adds to its appeal.
Platinum Compounds:
Platinum forms various compounds, with platinum(II) and platinum(IV) being the most common oxidation states. Notable compounds include platinum chloride, platinum oxide, and platinum sulfide, each exhibiting unique chemical characteristics.
Chemical Reactions with Other Elements:
Platinum's inert nature makes it resistant to corrosion and tarnish, ensuring its stability in various environments. It forms alloys with other metals, enhancing its strength and durability.
Occurrence and Production:
While platinum is relatively rare in the Earth's crust, it can be found alongside other precious metals like palladium and rhodium. Mining operations and recycling processes contribute to the global production of this coveted metal.
Uses and Facts:
Platinum's applications extend beyond its role in jewelry, finding use in catalytic converters, medical devices, electrical contacts, and more. Its scarcity and unique properties contribute to its high value in the market.
Platinum in Jewelry:
The use of platinum in jewelry dates back to ancient civilizations, and its popularity has only grown over time. Platinum rings, bracelets, and other accessories are prized for their durability, hypoallergenic properties, and timeless elegance. The metal's ability to withstand wear and tear makes it an ideal choice for crafting exquisite pieces that stand the test of time.
Platinum Rings and Love Bands:
Platinum's association with love and commitment has led to its prominence in the crafting of engagement rings and wedding bands. The purity of platinum symbolizes enduring love, making it a popular choice for couples seeking a timeless and meaningful connection.
Platinum Price and Market Trends:
The rarity of platinum, coupled with its diverse applications, influences its market price. Investors and enthusiasts closely monitor platinum prices, which can be influenced by factors such as industrial demand, economic conditions, and geopolitical events.
Why Platinum is Expensive:
Platinum's precious status can be attributed to a combination of its rarity, remarkable properties, and diverse range of applications. Here are key reasons why platinum is considered precious:
1. Rarity:
Platinum is one of the rarest elements on Earth. It is estimated that the annual production of platinum is only a fraction of that of gold and silver. The scarcity of platinum contributes significantly to its high market value.
2. High Melting and Boiling Points:
Platinum has exceptionally high melting and boiling points, making it resistant to heat and corrosion. This property is crucial in various industrial applications, such as catalytic converters in automobiles and high-temperature equipment, adding to its overall value.
3. Corrosion Resistance:
Platinum exhibits exceptional resistance to corrosion and tarnishing, maintaining its luster over time. This corrosion resistance is highly desirable, especially in jewelry, where durability and longevity are essential.
4. Catalytic Properties:
Platinum is an outstanding catalyst, meaning it can accelerate chemical reactions without being consumed in the process. This property is exploited in catalytic converters in automobiles, where platinum helps convert harmful pollutants into less harmful substances.
5. Versatility in Alloys:
Platinum forms strong and durable alloys with other metals, enhancing its versatility and making it suitable for a wide range of applications. Commonly, platinum is alloyed with metals like iridium, palladium, and rhodium to create materials with specific properties.
6. Biocompatibility:
Platinum's biocompatibility makes it suitable for medical applications, such as in pacemakers, dental work, and certain medical implants. The metal's inert nature reduces the risk of allergic reactions, making it an ideal choice for these critical applications.
7. Symbol of Luxury and Status:
The rarity and enduring beauty of platinum have made it a symbol of luxury and status. Its use in high-end jewelry, particularly in engagement rings and wedding bands, is often associated with timeless elegance and commitment.
8. Industrial Demand:
Platinum's unique properties, such as its catalytic abilities, electrical conductivity, and resistance to corrosion, contribute to its demand in various industrial sectors. This demand further amplifies its value in the market.
9. Investment Appeal:
Platinum is considered a precious metal along with gold, silver, and palladium. Many investors view platinum as a valuable asset and a hedge against economic uncertainties, contributing to its appeal in the investment market.
Conclusion:
In conclusion, platinum's unique combination of beauty and utility places it in a league of its own among precious metals. Whether adorning fingers in the form of rings or serving crucial roles in industrial applications, platinum continues to be a symbol of luxury, resilience, and enduring value. As we navigate the ever-changing landscape of commodities and fashion, the mystique of platinum remains unyielding, captivating the hearts and minds of those who appreciate its timeless allure.
Also Read:
Read about all 118 Elements, Symbols, Characteristics, Compounds and Uses








0 Comments