Atomic mass
Atomic mass
refers to the mass of an atom (ma or m). Although the unified atomic mass unit
(symbol: Da) is widely used to express atomic mass, the kilogramme (symbol: kg)
is the SI measure of mass (u). The mass of an unbound carbon-12 atom in its
ground state is 112 Da. Protons and neutrons make up almost all of an atom's
mass, with electrons and nuclear binding energy making up the majority of the
remaining mass. As a result, the mass number and the atomic mass have values
that are highly comparable. The atomic mass can be used to convert between mass
in kilogrammes and mass in daltons.
Atomic Mass Formula
By dividing the
atomic mass ma of an isotope by the atomic mass constant mu, one can derive the
relative isotopic mass, which is a dimensionless number.
Consequently, a carbon-12 atom's relative isotopic mass is 12 whereas its
atomic mass is 12 Da by definition. The relative molecular mass is the total of
all the atoms' respective isotopic masses.
A specific
isotope of an element is described by its relative isotopic mass and atomic
mass. The elemental atomic mass, which is the average (mean) atomic mass of an
element, weighted by the abundance of the isotopes, is useful since things are
typically not isotopically pure. The weighted mean relative isotopic mass of a
(average naturally occurring) mixture of isotopes is what is known as the
dimensionless (standard) atomic weight.
Due to binding
energy mass loss (per E = mc2), the atomic mass of atoms, ions, or atomic
nuclei is somewhat less than the total of the masses of its constituent
protons, neutrons, and electrons.
Atomic mass of
hydrogen is 1.00784 u & Atomic mass is oxygen is 8
Relative Isotopic Mass
Not to be
confused with the averaged quantity atomic weight (see above), which is an
average of values for many atoms in a particular sample of a chemical element,
is relative isotopic mass, a feature of a single atom.
While relative isotopic mass has no dimensions and no units, atomic mass is an absolute quantity. Relative isotopic mass refers to this scaling in relation to carbon-12, as shown by the word "relative" in the name. This loss of units is caused by the employment of a scaling ratio with respect to a carbon-12 reference.
The mass of a
particular isotope (more specifically, any single nuclide) multiplied by the
mass of carbon-12, where the latter must be established experimentally, yields
the relative isotopic mass. The mass of an isotope or nuclide relative to 1/12
of the mass of a carbon-12 atom is equivalently known as the relative isotopic
mass of that isotope or nuclide.
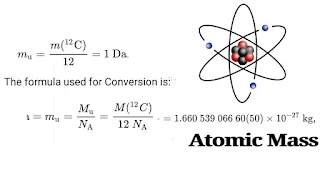
For instance, a carbon-12 atom's relative isotopic mass is precisely 12. For reference, a carbon-12 atom has an exact mass of 12 daltons. The atomic mass of a carbon-12 atom can also be stated in any other mass units, such as kg, where the value is 1.99264687992(60)10⁻²⁶ kg.
The relative
isotopic mass numbers of nuclides other than carbon-12 are not whole numbers,
but they are always close to whole numbers, just as the related atomic mass
when stated in daltons.
Relationship between atomic and molecular masses
Molecules have
definitions that are similar. By summing the atomic masses—not the conventional
atomic weights—of a compound's constituent atoms, one can determine the
molecular mass of the complex. On the other hand, the conventional atomic
weights are commonly used to calculate the molar mass (not the atomic or
nuclide masses). As a result, molar mass and molecular mass have somewhat
different numerical values and refer to distinct ideas. The total mass of a
molecule's individual atomic masses is known as the molecule's molecular mass.
Molar mass is the average of all the masses of the individual molecules that
make up an ensemble that is chemically pure but isotopically heterogeneous. In
both scenarios, it is necessary to account for the multiplicity of the atoms
(the number of times it occurs), which is often done by multiplying each unique
mass by the multiplicity.
History
John Dalton,
Thomas Thomson, and Jöns Jakob Berzelius were the first researchers to
calculate the relative atomic masses of atoms between 1803 and 1805, and
between 1808 and 1826. According to Prout's idea, which was put forth in the
1820s, all atomic masses would turn out to be precise multiples of hydrogen,
relative atomic mass (also known as atomic weight) was originally defined
relative to that of the lightest element, hydrogen, which was taken to be 1.00.
However, Berzelius quickly demonstrated that this wasn't even close to being
accurate; in fact, for some elements, like chlorine, the relative atomic mass,
at 35.5, is almost exactly halfway between two integral multiples of hydrogen.
Later, it was discovered that this was primarily caused by a mixture of
isotopes and that, to within about 1%, the atomic masses of pure isotopes, or nuclides,
are multiples of the mass of hydrogen.
By using
Avogadro's law, Stanislao Cannizzaro improved relative atomic masses in the
1860s (notably at the Karlsruhe Congress of 1860). By comparing the vapour
density of a group of gases with molecules containing one or more of the
chemical element in question, he developed a law to determine the relative
atomic masses of elements: the different amounts of the same element contained
in different molecules are all whole multiples of the atomic weight.
There were two
distinct atomic-mass scales used by chemists and physicists in the 20th
century, up to the 1960s. The natural mixture of oxygen isotopes had an atomic
mass of 16, according to the "atomic mass unit" (amu) scale used by
chemists, although the same number 16 was only given to the atomic mass of the
most prevalent oxygen isotope by physicists (16O, containing eight
protons and eight neutrons). However, as natural oxygen also contains oxygen-17
and oxygen-18, two distinct tables of atomic mass were required. The carbon-12,
or 12C,-based unified scale satisfied the physicists' requirement
that the scale be based on a pure isotope and was numerically comparable to the
scale used by chemists. As the "unified atomic mass unit," this was
chosen. The dalton and symbol "Da" are the current International
System of Units (SI) major recommendations for this unit's designation. The
accepted names and symbols for the same unit are "unified atomic mass
unit" and "u."
In the majority
of contemporary usage, the word "atomic weight" is gradually being
phased out in favour of the term "relative atomic mass." This change
in terminology, which dates back to the 1960s, has been the subject of intense
discussion in the scientific community. It was brought about by the adoption of
the unified atomic mass unit and the recognition that the term
"weight" wasn't quite accurate. The main justification for keeping
the term "atomic weight" was that it was well understood by experts
in the field, that it was already in use (in the sense that it is currently
defined), and that "relative atomic mass" could be mistaken for
relative isotopic mass (the mass of a single atom of a given nuclide expressed
dimensionlessly relative to 1/12 of the mass of carbon-12; see section above).
A secondary synonym for atomic weight called "relative atomic mass" was introduced in 1979 as a compromise. Twenty years later, the term "relative atomic mass" has replaced these synonyms as the preferred word.
The word
"standard atomic weights," which refers to the standardised expectation
atomic weights of various samples, has not been altered because the term
"standard relative atomic mass" would have been created by simply
replacing "atomic weight" with "relative atomic mass."
Also Read:
Resolver (Electrical) | Rotary Electrical Transformer | What is the purpose of a resolver?
Full Authority Digital Engine Control (FADEC) System Description & Operation










0 Comments