Unveiling Berkelium: The Element of Discovery
Berkelium:
In the vast expanse of the periodic table lies an element that often remains shrouded in mystery—berkelium. Named after the University of California at Berkeley, where it was discovered, berkelium (Bk) is a fascinating element with unique properties and a story that epitomizes the relentless pursuit of scientific exploration.
Discovery and Naming:
Berkelium was first synthesized by Stanley G. Thompson, Albert Ghiorso, Glenn T. Seaborg, and Kenneth Street Jr. in December 1949 at the University of California Radiation Laboratory. It was produced by bombarding americium-241 with alpha particles, resulting in the creation of berkelium-243. The element was named after the city and university where it was discovered, paying homage to the institution's significant contributions to nuclear chemistry.
Chemical Properties:
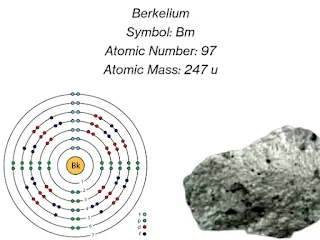
As a member of the actinide series, berkelium shares similarities with other elements in its group. It has an atomic number of 97, making it relatively unstable. Berkelium chemical elements has a most stable isotope, berkelium-247, it has a half-life of only 1,380 years. Due to its radioactive nature, berkelium is primarily studied for research purposes and has no practical applications outside scientific inquiry.
Physical Properties:
Berkelium is a silvery-white metal that tarnishes slowly in dry air and reacts with moisture. Its melting point is estimated to be around 986 degrees Celsius, and it exhibits magnetic properties below room temperature.
Electron Configuration and Valency:
The electron configuration of berkelium is [Rn] 5f9 7s2, Electron configuration in long form is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 5f9 6s2 6p6 7s. Being a member of the actinide series, berkelium typically exhibits a valency of +3, but it can also form compounds with other oxidation states, such as +4.
Berkelium Compounds and Reactions:
Berkelium forms compounds with various elements, primarily in the +3 oxidation state. Berkelium oxide (Bk₂O₃) and berkelium fluoride (BkF₃) are some examples. These compounds are mainly studied for their properties in nuclear research and are not utilized in practical applications.
In terms of reactions, berkelium exhibits typical behavior for actinide elements, engaging in various chemical reactions, primarily involving oxidation and reduction processes. However, due to its scarcity and radioactive nature, practical applications of these reactions are limited.
Occurrence and Production:
Berkelium is not found naturally on Earth and is exclusively produced through artificial means in nuclear reactors. It is a transuranic element, meaning it is synthesized by bombarding heavier elements with particles in controlled environments. The production process is complex and costly, contributing to berkelium's status as a rare and expensive element.
Uses and Facts:
Despite its scarcity and lack of practical applications, berkelium plays a crucial role in scientific research, particularly in nuclear physics and chemistry. Its unique properties make it valuable for studying nuclear reactions, the behavior of heavy elements, and the development of new materials.
Interestingly, berkelium has been used in the production of superheavy elements through nuclear fusion experiments. These endeavors aim to expand our understanding of the fundamental forces governing the universe and push the boundaries of scientific knowledge.
Conclusion:
In conclusion, berkelium stands as a testament to human curiosity and ingenuity. Its discovery and study exemplify the relentless pursuit of scientific exploration, driving us to unravel the mysteries of the universe and expand the boundaries of human knowledge.
As we continue to delve deeper into the realms of nuclear physics and chemistry, berkelium will undoubtedly remain a key player, offering insights into the fundamental building blocks of matter and the forces that shape our world.
Also Read:









0 Comments