Unveiling the Mysteries of Radium: A Glowing Journey through the Periodic Table
Radium:
In the vast expanse of the periodic table, certain elements stand out due to their unique properties and intriguing histories. One such element is radium – a fascinating member of the alkaline earth metals group. Join us on a journey as we explore the chemical intricacies, historical significance, and diverse applications of radium.
Radium Basics:
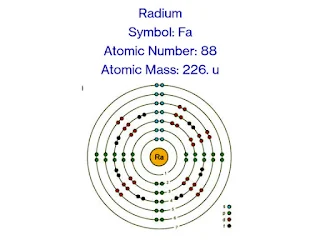
Symbol: Ra
Latin Name: Radium
Atomic Number: 88
Atomic Mass: Approximately 226 u/mol
Position in Periodic Table: Group 2, Period 7 (alkaline earth metals)
Electron Configuration: [Rn] 7s2
Electron Configuration
in long form: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 7s2
Valence Electron: 2
Valency: +2
Chemical and Physical Properties:
Radium, like its alkaline earth metal counterparts, is silvery-white in appearance. However, what sets it apart is its radioactivity. Discovered by Marie and Pierre Curie in 1898, radium exhibits a distinctive luminescence, emitting a faint blue glow in the dark.
Radium Compounds:
Radium forms various compounds, with radium chloride (RaCl₂) being one of the most notable. These compounds often display unique radioactive properties, contributing to their applications in medical and scientific fields.
Chemical Reactions:
Radium chemical element is highly reactive due to its position in the alkaline earth metals group. Radium readily reacts with water to form radium hydroxide and hydrogen gas. Additionally, it can react with non-metals and halogens, showcasing its versatile chemical behavior.
Occurrence and Production:
While radium is a relatively rare element, it is found in trace amounts in uranium and thorium ores. The production of radium typically involves the extraction from uranium ore residues, utilizing intricate chemical processes.
Uses and Applications:
1. Medical Applications: Radium has historically been used in cancer treatments due to its alpha-particle emissions. However, its use has diminished over the years with the development of safer alternatives.
2. Luminescent Paint: In the early 20th century, radium-based luminescent paints were employed for watch dials and aircraft instruments, as the radioactive glow provided visibility in low-light conditions.
3. Scientific Research: Radium's radioactive properties make it valuable in scientific experiments, particularly in studies involving radioactivity and nuclear physics.
Facts and Trivia:
Radium element is the heaviest and most radioactive of the alkaline earth metals.
Its discovery by the Curies led to groundbreaking advancements in the understanding of radioactivity.
Marie Curie, who isolated radium, was the first woman to win a Nobel Prize and remains an iconic figure in the history of science.
Conclusion:
Radium, with its captivating glow and radioactive allure, occupies a special place in the periodic table. From its discovery by the Curies to its applications in medicine and industry, this element has left an indelible mark on science and technology. While its use has evolved over time, radium's legacy as a pioneer in the realm of radioactivity continues to inspire curiosity and exploration.
Also Read:









0 Comments