Exploring Mendelevium: Unraveling the Mysteries of Element 101
Mendelevium:
In the vast and intricate landscape of the periodic table lies a realm of elements waiting to be discovered, each with its own unique properties and characteristics. Among these elements stands Mendelevium, a fascinating and elusive member of the actinide series. Named after the eminent Russian chemist Dmitri Mendeleev, who is renowned for his contributions to the periodic table, Mendelevium holds a special place in the realm of chemistry. Let's embark on a journey to unravel the mysteries of this remarkable element.
Discovery and Naming
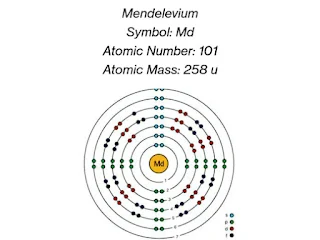
Mendelevium, with the chemical symbol Md and atomic number 101, Atomic mass 258 u, was first synthesized in 1955 by a team of scientists led by Albert Ghiorso at the University of California, Berkeley. The element was created by bombarding einsteinium-253 atoms with alpha particles, resulting in the formation of mendelevium-256.
In honor of Dmitri Mendeleev, the pioneering scientist who laid the groundwork for the modern periodic table, element 101 was named Mendelevium. Mendeleev's visionary contributions to chemistry provided the framework for organizing the elements based on their atomic properties, making it fitting to immortalize his name in the world of elemental discovery.
Properties and Characteristics
Mendelevium is a radioactive metal, and due to its high atomic number and unstable nature, it is extremely rare and challenging to study. As an actinide element, it shares similarities with its neighboring elements such as actinium, thorium, and uranium.
In its most stable form, mendelevium-258, this element has a half-life of approximately 51 days. Its physical properties, such as melting and boiling points, are not precisely known due to the minuscule amounts of mendelevium that can be produced and isolated.
Electron configuration and Valency
Electron configuration: [Rn] 5f13 7s2
Eelectron configuration in long form: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 5f13 6s2 6p6 7s2..
Placement in the seventh period of the periodic table. With a valency that can vary depending on its chemical environment, mendelevium primarily exhibits a +3 oxidation state in compounds.
Compounds and Chemical Reactions
Due to its limited availability and highly radioactive nature, the study of mendelevium compounds is exceptionally challenging. However, researchers have managed to synthesize a few compounds, primarily in trace amounts for experimental purposes.
One notable compound is mendelevium dioxide (MdO₂), which shares similarities with other actinide dioxide compounds. These compounds typically exhibit high melting points and are insoluble in water.
In terms of chemical reactions, mendelevium primarily interacts with other elements through processes such as nuclear transmutation and radioactive decay. Its reactivity is largely influenced by its position in the periodic table and its unstable nuclear configuration.
Occurrence and Production
Mendelevium is not found naturally on Earth and is instead produced through artificial means in nuclear reactors or particle accelerators. The primary method involves bombarding heavier actinide elements, such as einsteinium or fermium, with high-energy particles to induce nuclear reactions that lead to the formation of mendelevium isotopes.
Due to the complexity and expense of the production process, only minute quantities of mendelevium have ever been synthesized. As a result, it remains one of the rarest and most elusive elements known to humankind.
Uses and Applications
Owing to its scarcity and highly radioactive nature, mendelevium has no practical applications outside of scientific research. However, its study provides valuable insights into nuclear structure, radioactive decay processes, and the behavior of heavy elements.
Researchers utilize mendelevium primarily as a tool for fundamental research in nuclear physics and chemistry. Its unique properties make it a valuable subject for investigating the behavior of heavy nuclei and expanding our understanding of the periodic table.
Fascinating Facts
- Mendelevium is one of the few elements named after a specific individual, paying homage to Dmitri Mendeleev's contributions to chemistry.
- The discovery of mendelevium marked another milestone in the ongoing quest to explore and understand the properties of heavy elements.
- Due to its fleeting existence and extreme rarity, mendelevium remains a tantalizing challenge for scientists seeking to unlock the secrets of the atomic realm.
Conclusion
In conclusion, Mendelevium stands as a testament to human ingenuity and the boundless curiosity that drives scientific exploration. As we continue to push the boundaries of our knowledge, this enigmatic element serves as a reminder of the wonders that await discovery within the hidden depths of the periodic table.
Also Read:









0 Comments